|
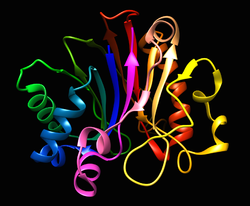
Figure 1 - Functional domains in the Klenow Fragment (left)
and DNA Polymerase I (right). |
DNA
polymerase I is an enzyme that participates in the DNA replication of
prokaryotes. It is the predominant and most abundant polymerizing enzyme found
in E.coli. It was the first DNA polymerase
to be identified and discovered by Arthur Kornberg, an American biochemist in
1956. He was able to characterize the enzyme initially in E.coli. In E.coli the
enzyme is a single polypeptide chain comprised of 928 aminoacids and it has a
molecular weight of 109kDa. Nevertheless, the enzyme does not occur only in E.coli but also in many prokaryotes. In
prokaryotes it is mostly encoded by polA
gene.
Uses:
It has three
distinct functions: 3′ to 5′ exonuclease or “proofreading” function because it
can remove a nucleotide from the 3’ terminus which was incorrectly inserted in
the DNA strand, 5′ to 3′ exonuclease and 5′ to 3′ polymerase.
The second
function is the 5’ to 3’ exonuclease. This enzyme has the ability to remove
nucleotides from the 5’ terminus. Furthermore, the nucleotides removed can be
either of the deoxyribonucleotides or ribonucleotides. The main goal of the 5′
to 3′ exonuclease activity is to remove ribonucleotide primers that are used in
DNA replication.
The 5’ to 3’
polymerase is the main activity of the enzyme and it means that it can add
nucleotides to the 3’-OH terminus of the previous nucleotide in a DNA template.
In order for polymerase to start adding nucleotides it needs a primer (consisted
of approximately 10 ribonucleotides) to begin DNA synthesis. DNA pol I adds
approximately 15-20 nucleotides per second.
Common
reaction buffer:
A common reaction buffer is
Tris-HCl (pH 7.2).
References:
ScienceDirect, DNA
Polymerases,, viewed in November 13, 2018, https://www.sciencedirect.com/topics/neuroscience/dna-polymerase-i
Worthington Biochemical Corporation, DNA Polymerase I, viewed in
November 13, 2018, http://www.worthington-biochem.com/DNAECI/default.html
Biology Online, DNA
Polymerase I, viewed in November 13, 2018, https://www.biology-online.org/dictionary/DNA_polymerase_I
Technical information of kit M205A, DNA polymerase I, Promega
Corporation.
Evangelia Nikou (e8749) | Hugo Rodrigues (a85946) | Mélanie Pereira (a83980)
Pedro Gonçalves (a84784) | Ricardo Fernandes (a86254)
University of Minho | School of Sciences | Department of Biology
Degree in Applied Biology | Genes and Genomes